Metal alloys
I can evaluate the metallic structure model in terms of its ability to explain some physical properties of metals and metal alloys.
Metal alloys
I can evaluate the metallic structure model in terms of its ability to explain some physical properties of metals and metal alloys.
Link copied to clipboard
These resources will be removed by end of Summer Term 2025.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
These resources were created for remote use during the pandemic and are not designed for classroom teaching.
Lesson details
Key learning points
- In pure metals, ions are arranged in a regular structure with delocalised electrons between them
- If a metal is stretched or forced out of shape, its ions are able to move position without the metallic bonds breaking
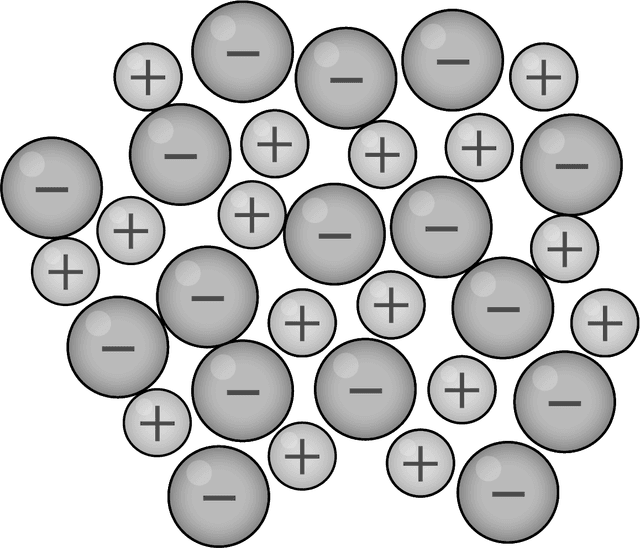
- An alloy is a mixture of different metal elements or of metals with non metal elements (i.e. carbon)
- Ions of different metal elements may be different sizes and have different electrical charges
- The structure of an alloy is not as regular as a pure metal and this affects its properties
Keywords
Alloy - An alloy is a mixture of two or more elements, where at least one element is a metal.
Physical properties - A physical property is a characteristic of a substance that can be observed or measured. For example, the temperature at which a substance melts.
Delocalised electron - Electrons are said to be delocalised when they are free to move through the structure of a metal and can carry an electrical current.
Molten - Molten is the term used to describe a liquid substance formed by heating solid metals, glass, or rocks.
Common misconception
The metallic structure model is a replication of reality. Therefore, metals contain seawater. The concept of electric current is challenging. Some may think that electrons jump from atom to atom.
Discuss why scientists use models with the pupils, explaining that a model is only as good as what it can explain, so it develops over time. Remind pupils how an electric circuit works before discussing why metals are good conductors.
To help you plan your year 10 chemistry lesson on: Metal alloys, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 chemistry lesson on: Metal alloys, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Structure and bonding unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Equipment
Licence
Starter quiz
6 Questions
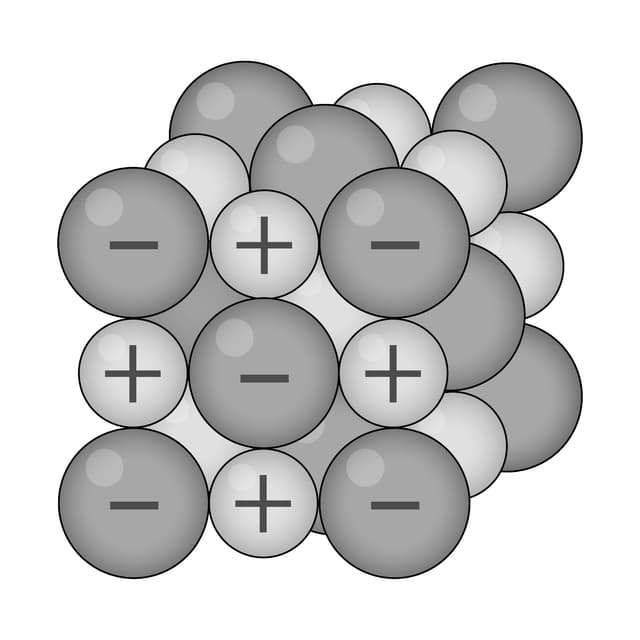
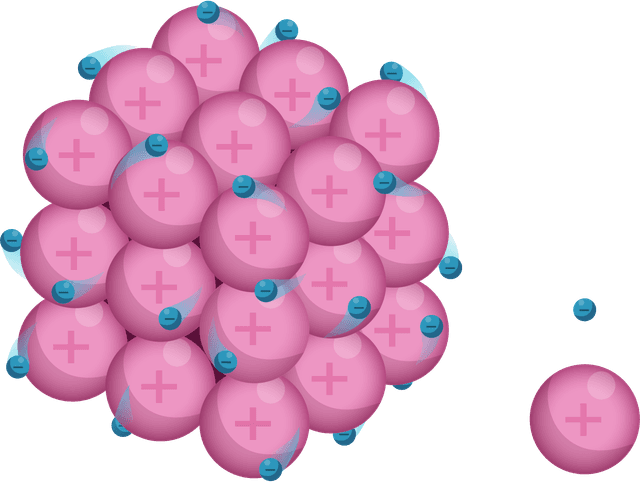
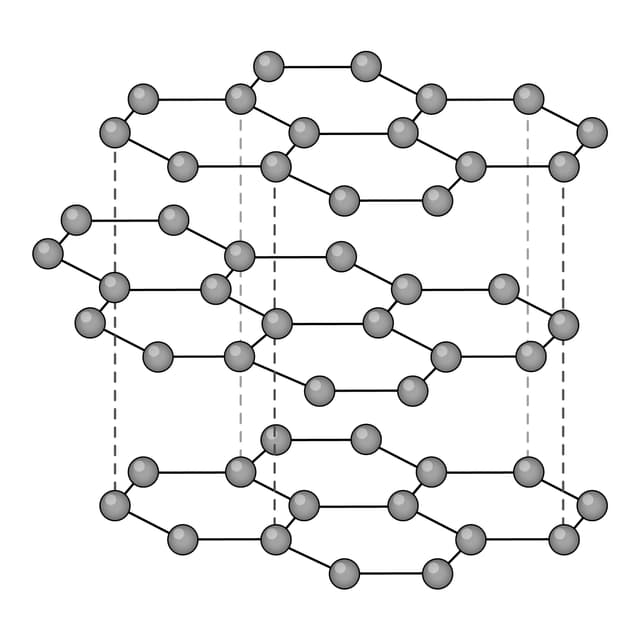
Ions are arranged in layers that can easily slide over each other.
Ions are surrounded by a 'sea' of delocalised electrons.
Strong electrostatic attraction between ions (+) and electrons (–).
Exit quiz
6 Questions
A mixture of two or more elements, at least one of which is a metal.
Negatively charged particle free to move through the metal structure.
A liquid substance formed by heating solid metal, glass or rocks.
A characteristic of a substance that can be observed or measured.


