Myths about teaching can hold you back
- Year 10
- AQA
- Higher
Breaking and making bonds: using bond energies
I can describe how bond making and breaking use or release energy, and use bond energies to calculate whether a reaction is exothermic or endothermic.
- Year 10
- AQA
- Higher
Breaking and making bonds: using bond energies
I can describe how bond making and breaking use or release energy, and use bond energies to calculate whether a reaction is exothermic or endothermic.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
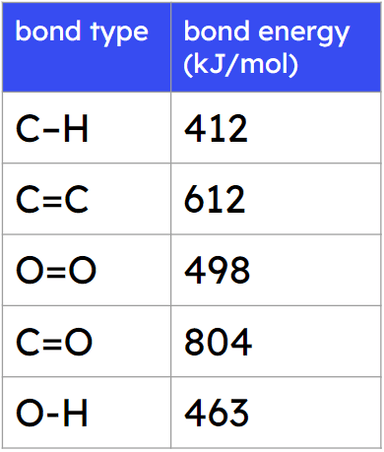
- The energy needed to break bonds and the energy released when bonds are formed can be calculated from bond energies.
- The difference between the energy needed to break bonds and the energy released is the overall energy change.
Keywords
Endothermic - An endothermic chemical reaction is a type of reaction in which energy is transferred from the surroundings to the products, e.g. photosynthesis.
Exothermic - An exothermic chemical reaction is a type of reaction in which energy is transferred from the reactants to the surroundings, e.g. combustion.
Activation energy - The minimum energy that the particles must have in order to react is known as the activation energy.
Bond energy - The energy required to break a covalent bond, or released when making a covalent bond. is known as the bond energy.
Common misconception
Pupils often make mistakes when identifying the different types of bonds and the number of each in a molecule. They may also reverse the calculation of the overall energy change, as make - break.
Molecules can be complex and should be drawn to show all the bonds. Emphasis that double and triple covalent bonds are different to single bonds. Pupils should be instructed to consider whether a reaction is exothermic or endothermic.
To help you plan your year 10 chemistry lesson on: Breaking and making bonds: using bond energies, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 chemistry lesson on: Breaking and making bonds: using bond energies, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Energy changes in reactions unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Licence
Prior knowledge starter quiz
6 Questions
Q1.What does an energy profile diagram show?
Q2.What occurs during a chemical reaction?
Q3.What is an exothermic reaction?
Q4.What is an endothermic reaction?
Q5.What is activation energy?
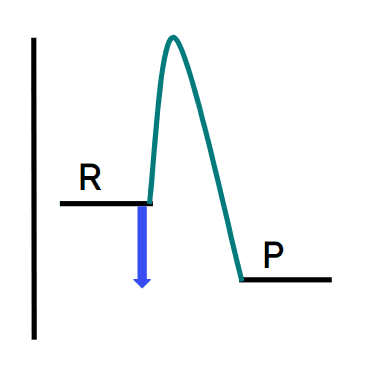
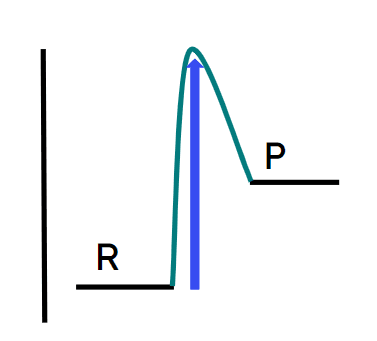
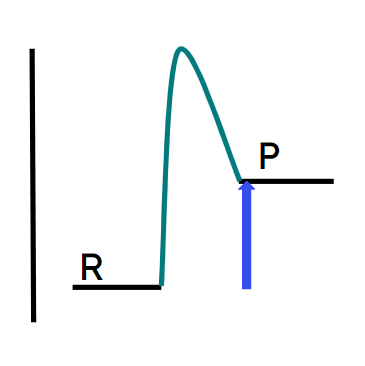
Q6.In which energy profile does the blue arrow show the energy change of an exothermic reaction?
Assessment exit quiz
6 Questions
Q1.The overall energy change for an exothermic reaction is...
Q2.Given the following data: reactants energy = 200 kJ, products energy = 300 kJ, what is the overall energy change? Is the reaction endothermic or exothermic?
Q3.Given the following data: reactants energy = 400 kJ, products energy = 250 kJ, what is the overall energy change? Is the reaction endothermic or exothermic?
Q4.During the combustion of methane, the reactants have an energy of 500 kJ and the products have an energy of 300 kJ. What is the overall energy change? Is the reaction endothermic or exothermic?
Q5.How many C-H bonds are there in the reactants and in the products of this reaction?
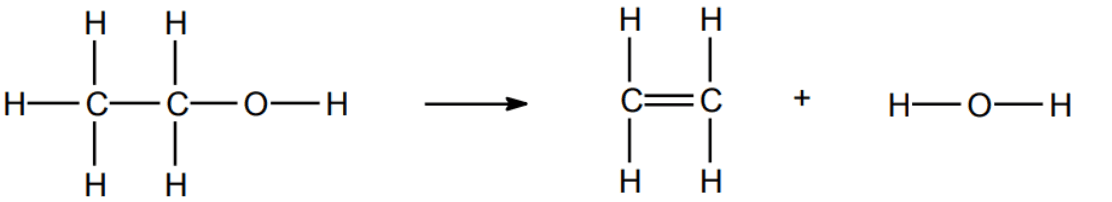
Q6.What is the overall energy change when ethanol (C₂H₅OH) reacts to form ethene (C₂H₄) and water (H₂O)? Give your answer in kJ/mol.