Myths about teaching can hold you back
- Year 11
- AQA
- Higher
- Year 11
- AQA
- Higher
Making ammonia
I can safely produce ammonia in the laboratory.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- Ammonia can be synthesised in the lab by heating a mixture of NH₄Cl and NaOH, producing NH₃ gas and other by-products.
- Ammonia is an alkaline gas that reacts readily with water to form a corrosive, basic solution.
- Ammonia gas turns moist red litmus paper blue, indicating its alkaline nature.
- Ammonia gas is soluble, toxic, and has a pungent smell, requiring careful handling.
- Ammonia reacts with hydrogen chloride (from concentrated hydrochloric acid) to form white fumes of ammonium chloride.
Keywords
Ammonia - a simple molecular substance, with the formula NH₃, is a soluble, toxic, pungent gas at room temperature.
Alkaline - a base that dissolves in water; these solutions have a pH greater than 7
Pungent - a description of a substance that has a sharply strong smell
Toxic - a substance that is poisonous, and can cause harm or death to living organisms
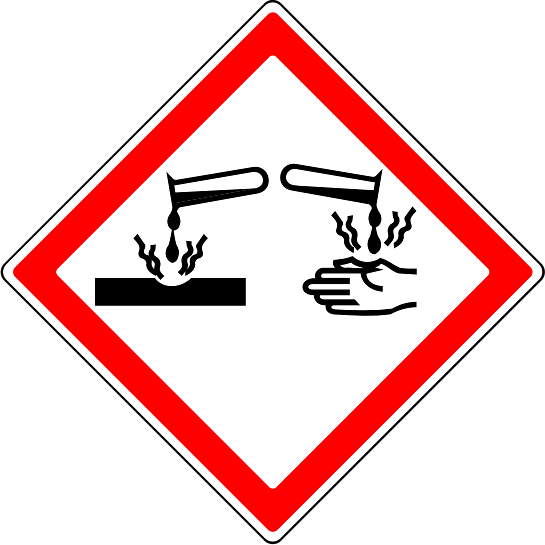
Corrosive - a substance that causes damage by chemical action, often resulting in severe injury or destruction
Common misconception
Pupils may think ammonia is safe to handle without precautions because it's a common household chemical.
Emphasise ammonia's toxicity and the importance of using PPE and proper ventilation.
To help you plan your year 11 chemistry lesson on: Making ammonia, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Making ammonia, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Industrial chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Equipment
ammonium chloride, calcium hydroxide, calcium oxide, concentrated hydrochloric acid, red and blue litmus paper, UI paper, access to fume cupboard, nitrile gloves, demo setup shown in slides
Content guidance
- Risk assessment required - equipment
Supervision
Adult supervision required
Licence
Prior knowledge starter quiz
6 Questions
Q1.Which of the following statements about bases are correct?
Q2.Nitrogen can be found in Group 5, period 2 of the periodic table. What does this tell you about the electron configuration in nitrogen?
Q3.What term do we use in science to show that a substance is dissolved in water?
Q4.Ammonium chloride has the formula NH₄Cl. Which of the following statements about the formula for ammonium chloride are correct?
Q5.What type of bonding forms an ammonia, NH₃, molecule?
Q6.Which of the following are examples of physical properties of a substance?
Assessment exit quiz
6 Questions
Q1.Match the following terms to the correct definition.
A poisonous substance that causes harm or death to living organisms.
A substance that causes damage by chemical action.
A base soluble in water producing a solution with a pH greater than 7.
Q2.Hydrogen chloride reacts with ammonia to form ammonium chloride, NH₄Cl. Which of the following chemical equations shows this reaction correctly?
Q3.What does this hazard warning symbol tell you about a substance?