Alcohols
I can describe and explain properties and reactions of alcohols.
Alcohols
I can describe and explain properties and reactions of alcohols.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
- Alcohols can react, at their functional group -OH, with sodium, oxygen, water, or an oxidising agent.
- Alcohols need less oxygen than equivalent alkanes in order to burn completely.
- Alcohols are good solvents which also mix with water.
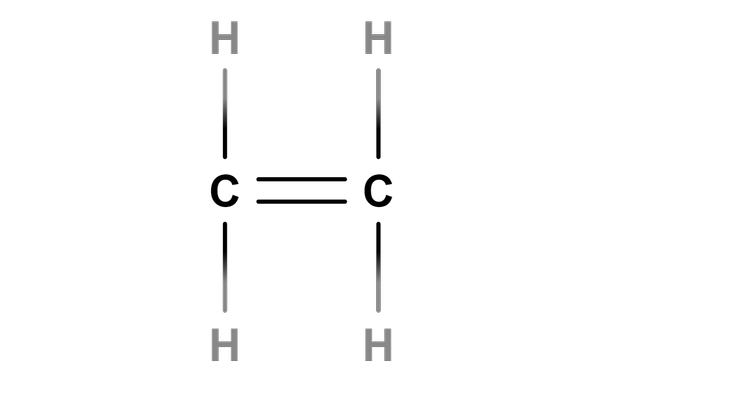
- The boiling point of ethanol is much higher than the boiling point of ethane.
- Ethanol can be formed via fermentation of glucose (renewable) or via hydration of ethene (non-renewable).
Keywords
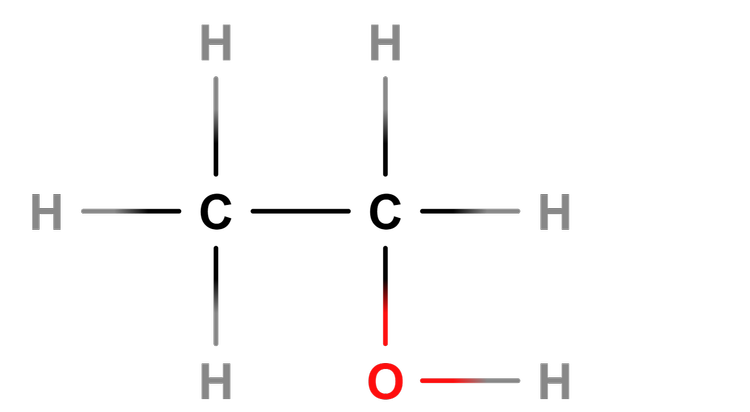
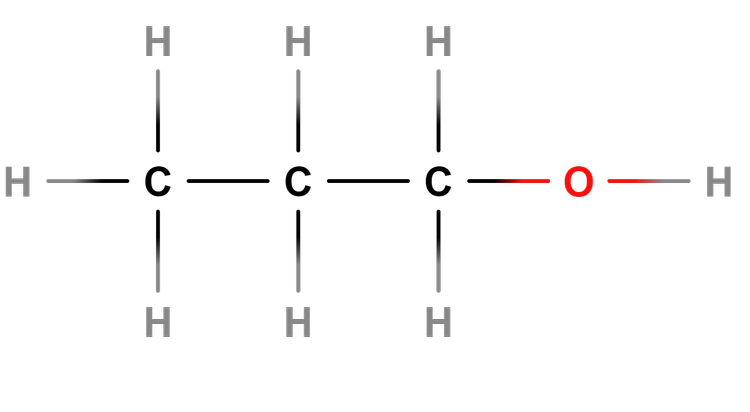
Alcohol - Alcohols are a homologous series of compounds that contain the -OH functional group.
Functional group - The functional group is the atom or group of atoms responsible for the way a compound reacts.
Solvent - A solvent is a liquid into which a solute dissolves.
Fermentation - Fermentation is a process that produces solutions of ethanol from sugar, resulting from the anaerobic respiration of microorganisms.
Hydration - A reaction involving the addition of water is known as hydration.
Common misconception
Incorrectly writing the molecular formula of alcohols.
Help pupils understand the difference between structural formula, e.g C₂H₅OH, and molecular formula, e.g C₂H₆O.
To help you plan your year 11 chemistry lesson on: Alcohols, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Alcohols, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Organic chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Equipment
Whoosh bottle resources - reaction vessel, rubber stopper, splint attached to metre ruler, and alcohols. Complete your own risk assessment.
Licence
Starter quiz
6 Questions
Exit quiz
6 Questions



