New
New
Year 11
Edexcel
Higher
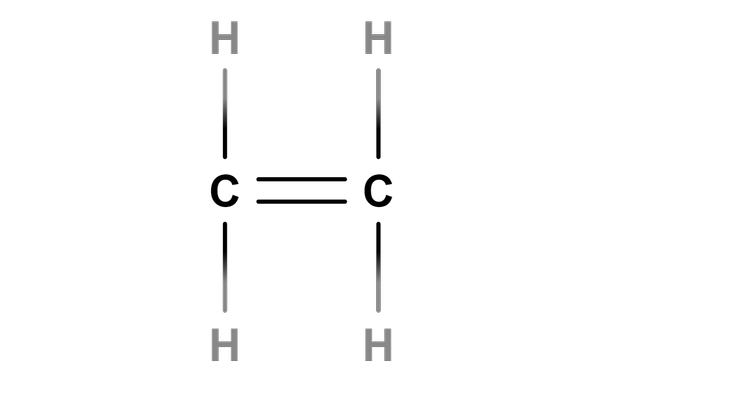
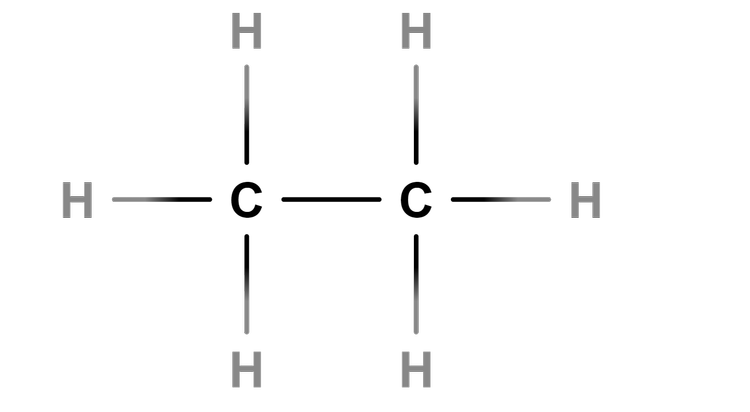
Chemical tests involving alkanes, alkenes and alcohols
I can use data to identify the functional group and predict the chain length for an alkane, alkene or alcohol.
New
New
Year 11
Edexcel
Higher
Chemical tests involving alkanes, alkenes and alcohols
I can use data to identify the functional group and predict the chain length for an alkane, alkene or alcohol.
These resources will be removed by end of Summer Term 2025.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
These resources were created for remote use during the pandemic and are not designed for classroom teaching.
Lesson details
Exit quiz
Download exit quiz