Myths about teaching can hold you back


- Year 11•
- OCR•
- Higher
Industrial equilibria: Contact Process
I can describe the Contact Process for the production of sulfuric acid, explain the importance of each step, and describe the conditions used.


- Year 11•
- OCR•
- Higher
Industrial equilibria: Contact Process
I can describe the Contact Process for the production of sulfuric acid, explain the importance of each step, and describe the conditions used.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- The Contact Process involves the production of sulfur trioxide (SO₃) from sulfur dioxide (SO₂) using a catalyst.
- The process has three main stages: burning sulfur to produce SO₂, converting SO₂ to SO₃, then converting SO₃ to H₂SO₄.
- The conversion of SO₂ to SO₃ is a reversible reaction performed at 1-2 atmospheres and 450°C, using a V₂O₅ catalyst.
- Sulfuric acid production is energy-intensive and releases large amounts of CO₂, contributing to global emissions.
- Sulfuric acid is vital for various industries, such as: fertilisers, chemical manufacturing, and petroleum refining.
Keywords
Raw material - The starting materials used to make products are known as the raw materials.
Feedstock - Refers to raw materials used in chemical processes to produce other substances.
Contact process - An industrial method for producing sulfuric acid by oxidising sulfur dioxide to sulfur trioxide.
Exothermic - A type of reaction in which energy is transferred from the reactants to the surroundings.
Corrosive - A substance that causes damage by chemical action, often resulting in severe injury or destruction.
Common misconception
Students often assume that the production of sulfuric acid in the Contact Process is a one-step process (similar to the Haber process).
Clarify that the Contact Process involves multiple stages, each requiring specific conditions. Emphasise the importance of these conditions in maximising yield and minimising energy consumption.
To help you plan your year 11 chemistry lesson on: Industrial equilibria: Contact Process, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Industrial equilibria: Contact Process, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Industrial chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Equipment
None required.
Licence
Prior knowledge starter quiz
6 Questions
Q1.In research labs high, pressure apparatus is contained in small spaces to reduce risk. The large of industry requires strong equipment as safety measures, to protect workers and the public.
Q2.Sulfuric acid is corrosive. What warning symbol would you expect to see on a flask of concentrated sulfuric acid?
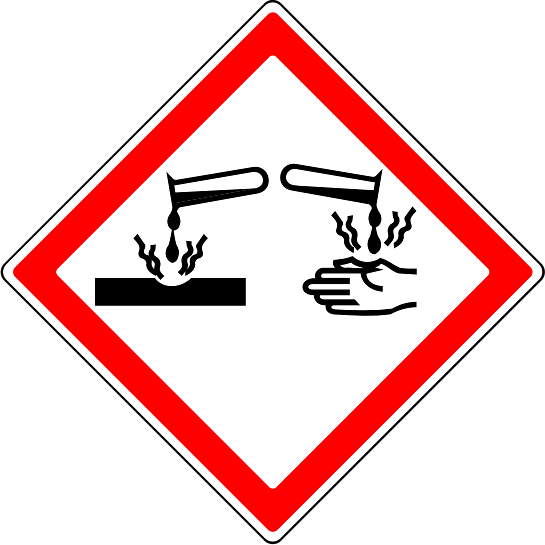
Q3.A type of reaction in which energy is transferred from the reactants to the surroundings is called an reaction.
Q4.Making sulfuric acid in industry has environmental as well as economic implications. Which of these are environmental (positive as well as negative)?
Q5.All feedstocks are raw materials, but not all raw materials are feedstock. Which statements explain the difference?
Q6.Many chemicals, including ammonia and sulfuric acid, are found in nature but in small quantities. Which of the following statements drive industrial chemistry?
Assessment exit quiz
6 Questions
Q1.Match the following terms with the correct definitions.
Contact process -
industrial method making sulfuric acid from sulfur, oxygen and water
corrosive -
causing severe injury, damage or destruction by chemical action
exothermic reaction -
reaction in which energy is transferred from reactants to surroundings
feedstock -
raw materials used in chemical processes to make other substances
Haber process -
industrial method for making ammonia from nitrogen and hydrogen
raw materials -
any starting materials used to make any kind of products

