Myths about teaching can hold you back


- Year 11•
- OCR•
- Higher
Industrial equilibria: Haber Process
I can describe the Haber Process for the production of ammonia, explain the significance of equilibrium in this industrial process, and describe the conditions used.


- Year 11•
- OCR•
- Higher
Industrial equilibria: Haber Process
I can describe the Haber Process for the production of ammonia, explain the significance of equilibrium in this industrial process, and describe the conditions used.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- In a reversible reaction the desired product needs to be continuously removed from the reaction.
- Natural gas is a feedstock for industrial production of ammonia.
- Hydrogen, one of the raw materials in ammonia production, is produced from natural gas and steam.
- In industry, the Haber process is typically performed at 150-300 atmospheres, 400-450°C, with an iron catalyst.
- Ammonia production is energy-intensive and releases large amounts of carbon dioxide, contributing to global emissions.
Keywords
Natural gas - A mixture of gases which are rich in hydrocarbons, consisting largely of methane.
Feedstock - Refers to the raw materials used in chemical processes to produce other substances.
Raw material - The starting materials used to make products are known as the raw materials.
Haber process - An industrial method for producing ammonia from nitrogen and hydrogen.
Common misconception
Students find it difficult to understand why continuous removal of ammonia is required to increase yield.
Highlight that the Haber Process is a dynamic equilibrium. If ammonia is not continuously removed, the equilibrium will shift back towards the reactants, reducing the yield.
To help you plan your year 11 chemistry lesson on: Industrial equilibria: Haber Process, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Industrial equilibria: Haber Process, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Industrial chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Equipment
None required.
Licence
Prior knowledge starter quiz
6 Questions
Q1.Which of these statements are required for a dynamic equilibrium to be established?
Q2.The direction of reversible reactions can be influenced by changing conditions, e.g. an exothermic reaction is favoured by lowering the temperature, but at the same time the reaction rate .
Q3.True or false? At dynamic equilibrium all bonds between all atoms need to be broken and new bonds need to be formed.
Q4.Consider this reaction:
CaCO$$_3(s)$$ ⇌ CaO$$_(s)$$ + CO$$_2(g)$$
What would happen if this was done in an open system?
Q5.Industrial chemistry is different from chemistry in a school lab or a research lab. The of the work is larger; therefore the risks and safety measures are larger too.
Q6.What is wrong with the graph below?
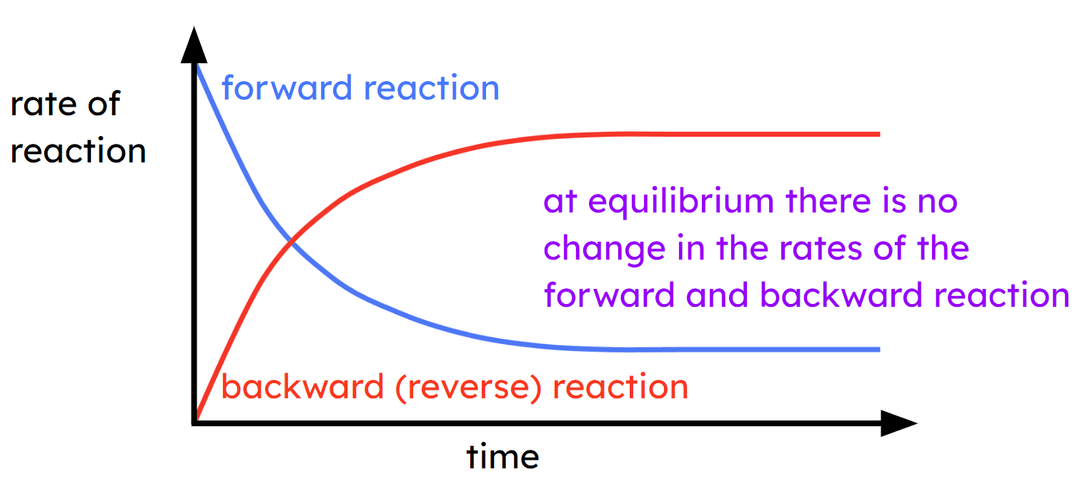
Assessment exit quiz
6 Questions
Q1.Traditional ammonia produced through the Haber process is called grey ammonia. Match the following terms with the correct definitions.
blue ammonia -
traditional ammonia, but involving carbon capture of the emissions
green ammonia -
ammonia produced through 100% renewable and carbon-free processes

