Carboxylic acids and esters
I can describe the structure of the first four carboxylic acids, and recognise and describe reactions where carboxylic acids are involved.
Carboxylic acids and esters
I can describe the structure of the first four carboxylic acids, and recognise and describe reactions where carboxylic acids are involved.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
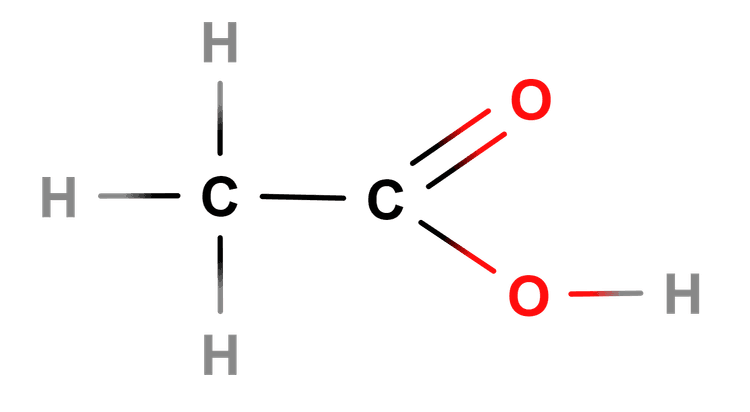
- Carboxylic acids have a -COOH functional group.
- Carboxylic acids are weak acids.
- When ethanol is oxidised, ethanoic acid is formed.
- Carboxylic acids react with carbonates to produce a metal salt, carbon dioxide and water.
- Carboxylic acid can react with alcohol to produce esters (such as ethyl ethanoate).
Keywords
Carboxylic acids - Carboxylic acids are a homologous series of compounds that contain the -COOH functional group.
Weak acids - Weak acids partially dissociate to form Hᐩ ions in solution.
Carbonate - A carbonate is a compound containing the CO₃²ᐨ ion. An example is calcium carbonate CaCO₃.
Esters - Esters are a homologous series of compounds that contain the -COO- functional group.
Ethyl ethanoate - Ethyl ethanoate is an ester produced from the alcohol ethanol and the carboxylic acid ethanoic acid.
Common misconception
Confusing the concentration and strength of acids.
Clearly define a strong and weak acid.
To help you plan your year 11 chemistry lesson on: Carboxylic acids and esters, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Carboxylic acids and esters, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Organic chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Licence
Starter quiz
6 Questions
C=C
OH
COOH
Exit quiz
6 Questions

A homologous series of compounds containing the -COOH functional group
Partially dissociate to form Hᐩ ions in solution
A compound containing the CO₃²ᐨ ion.
A homologous series of compounds containing the -COO- functional group


