Chemical tests involving alkanes, alkenes and alcohols
I can use data to identify the functional group and predict the chain length for an alkane, alkene or alcohol.


Chemical tests involving alkanes, alkenes and alcohols
I can use data to identify the functional group and predict the chain length for an alkane, alkene or alcohol.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
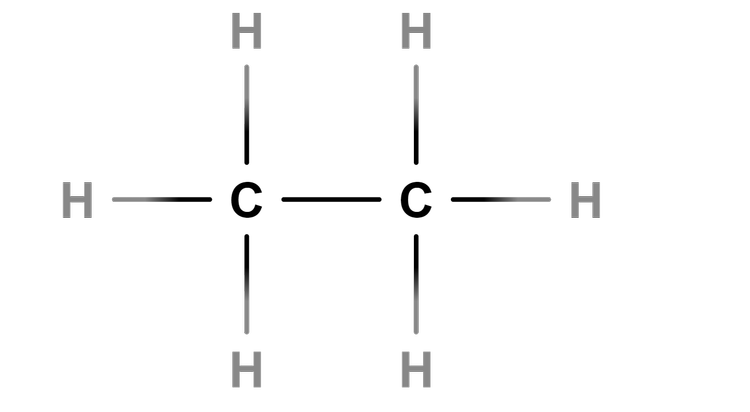
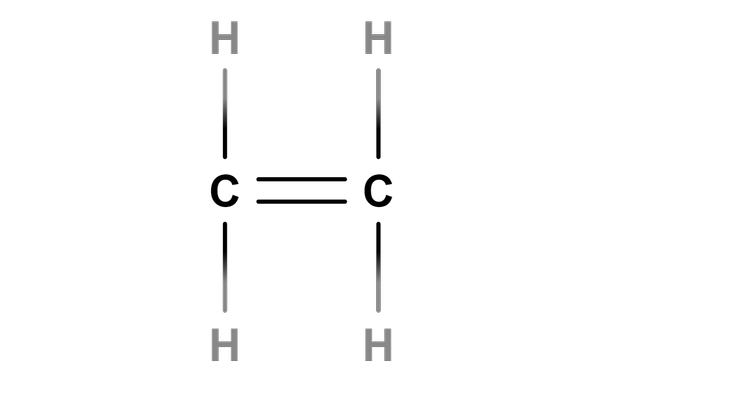
- A chemical test can identify the differences between alkanes and alkenes.
- Data from calorimetry and viscosity experiments can be used to predict the chain length of alkanes, alkenes & alcohols.
- As chain length increases, more energy is released during combustion.
- As chain length increases, the viscosity of alkanes, alkenes and alcohols increases.
Keywords
Homologous series - A homologous series of organic compounds have the same general formula, and show similar chemical properties and physical property trends.
Functional group - The functional group is the atom or group of atoms responsible for the way a compound reacts.
Calorimetry - Calorimetry is an experiment that can be used to measure the energy released from a fuel.
Viscosity - Viscosity is how easily a liquid flows. The higher the viscosity, the less easily a liquid flows.
Common misconception
Describing the colour change of bromine water with an alkene from orange to clear.
Coloured solutions can be clear if you can see through them. Colourless means it has no colour. Bromine water in the presence of an alkene changes from orange to colourless.
To help you plan your year 11 chemistry lesson on: Chemical tests involving alkanes, alkenes and alcohols, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 chemistry lesson on: Chemical tests involving alkanes, alkenes and alcohols, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 chemistry lessons from the Organic chemistry unit, dive into the full secondary chemistry curriculum, or learn more about lesson planning.

Licence
Starter quiz
6 Questions
alkene -
C=C
alcohol -
–OH
carboxylic acid -
–COOH
ester -
–COO–
Exit quiz
6 Questions