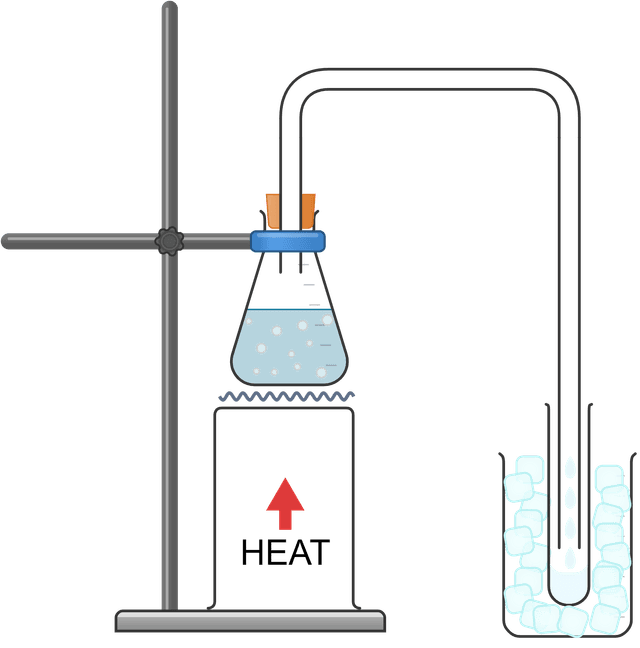
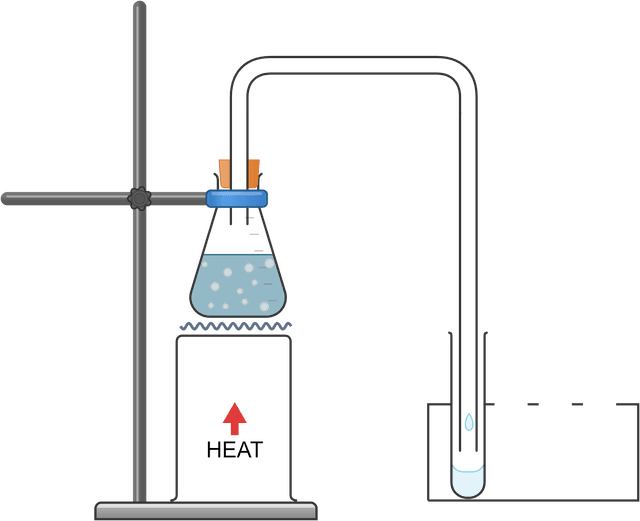
Distillation: separating a mixture of inks
I can describe how to successfully separate the components of ink.
Distillation: separating a mixture of inks
I can describe how to successfully separate the components of ink.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
- Appropriate equipment should be used to separate substances in a mixture of inks.
- Inks are a mixture of substances that can be separated using distillation.
- Care should be taken when heating substances to their boiling points.
- Simple distillation completed in the school lab has limitations.
Keywords
Component - a part of something, e.g. a tyre on a car
Distillation - a separation technique that uses boiling and condensation to remove and isolate a liquid component of a mixture
Delivery tube - a thin tube used to transfer (i.e. "deliver") a substance (usually in the gas state) from one container to another
Condenser - a piece of apparatus composed of a tube surrounded by a cold layer, usually cold water
Distillate - the liquid that is condensed from the gas state during distillation
Common misconception
Heating a mixture strongly will cause distillation to occur more quickly.
Heating a mixture strongly could cause multiple components to enter a condenser (or delivery tube), resulting in a contaminated distillate. Careful, gentle heating of the mixture to ensure no bubbles near the condenser will reduce contamination.
To help you plan your year 10 combined science lesson on: Distillation: separating a mixture of inks, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Distillation: separating a mixture of inks, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the Separating substances unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Equipment
Per group: conical flask, delivery tube, test tube, ice, beaker, inky water sample, heatproof mat, Bunsen burner, tripod, gauze
Content guidance
- Risk assessment required - equipment
Supervision
Adult supervision required
Licence
Starter quiz
6 Questions
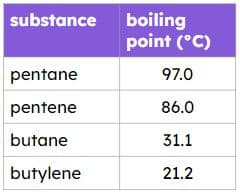
Exit quiz
6 Questions
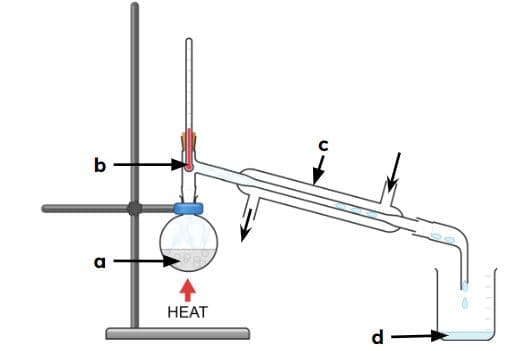
mixture
thermometer
condenser
distillate