Myths about teaching can hold you back
- Year 10
- AQA
- Foundation
Heating and cooling curves
I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance.
- Year 10
- AQA
- Foundation
Heating and cooling curves
I can explain the shape of a heating/cooling curve by describing the energy changes through the heating/cooling of a substance.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- An experiment can be planned to measure the temperature change through continuous heating of a substance
- Energy is needed to overcome forces of attraction between particles so temperature does not change when changing state
- The change in state of pure substances can be seen on a heating/cooling curve as a horizontal line
- There is a change of energy, arrangement, and organisation of particles of a pure substance at each stage
Keywords
Heating curve - shows temperature changes of a substance over time as it is heated.
Cooling curve - shows temperature changes of a substance over time as it is cooled.
Intermolecular forces - are forces between particles.
Common misconception
Pupils struggle to understand what is taking place at the horizontal parts of the graph.
Be concise with your language and ask pupils to use the same language. 'have enough energy to overcome the forces of attraction between particles', 'to slide past each other', 'to move past each other'.
To help you plan your year 10 combined science lesson on: Heating and cooling curves, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Heating and cooling curves, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the States of matter unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Licence
Prior knowledge starter quiz
6 Questions
Q1.Sort the states of matter and the temperatures at which changes of state occur into the correct order, starting with a substance in the solid state.
Q2.Freezing and condensing are exothermic processes. Melting, evaporation and boiling are processes.
Q3.A block of pure ice is heated in a beaker until it has all melted. Which of the following statements are correct?
Q4.Match the following keywords to their definition.
change from a solid state to a liquid state
change from a liquid state to a gas state
change from a liquid state to a solid state
change from a gas state to a liquid state
Q5.Which of the following statements describe what happens when a pure substance boils and changes from the liquid state to the gas state?
Q6.Which of the following statements describes what happens when a pure substance freezes and changes from the liquid state to the solid state?
Assessment exit quiz
6 Questions
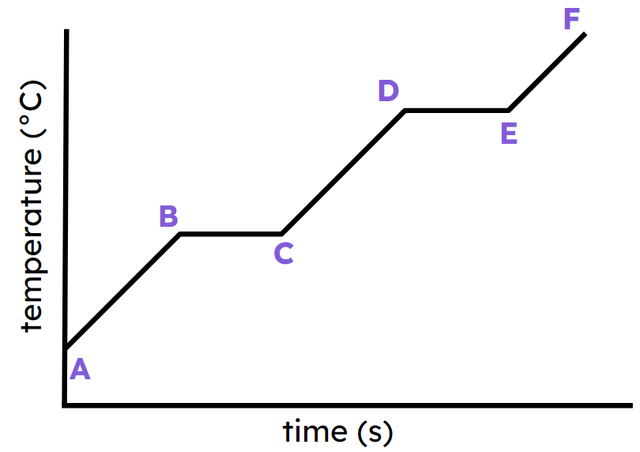
Q1.Consider a heating curve (see image). The following statements are about some of the sections (e.g. ‘CD’ means the sloping part from C to D). Which of the statements are correct?
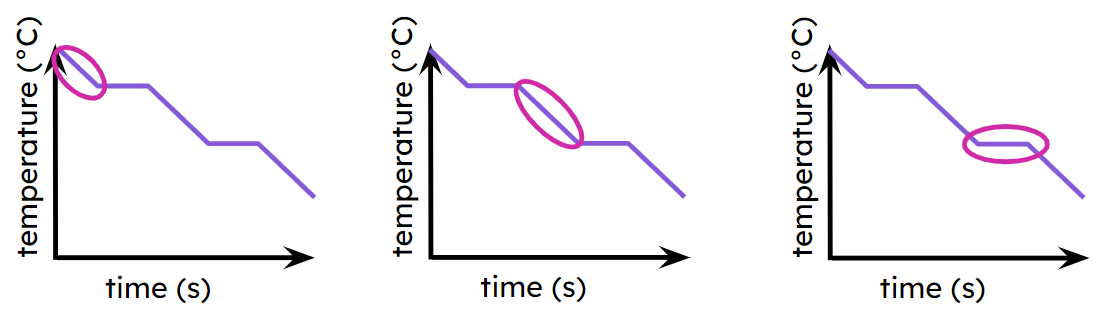
Q2.The image shows three cooling curves. What do the ringed sections represent, in order from left to right?
Q3.Sort the following stages of a heating curve into the correct order, starting from a statement about the solid substance:
Q4.In a heating curve experiment, the dependent variable is temperature, and the independent variable is .
Q5.Which of the following pieces of equipment would you use to measure the dependent variable in the heating curve experiment?
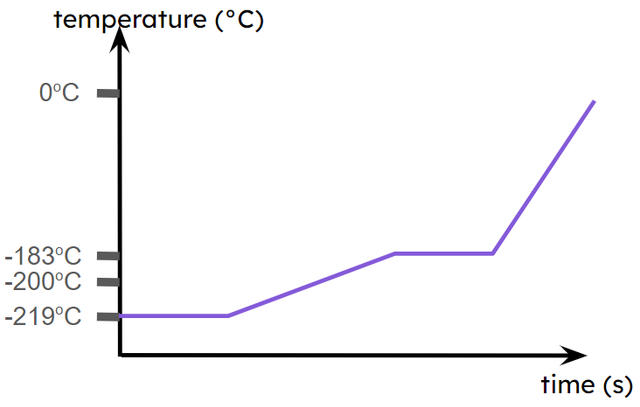
Q6.The image shows a curve for oxygen. This is a , although it starts at the melting point rather than with the substance in the solid state.