Myths about teaching can hold you back


- Year 10•
- AQA•
- Foundation
Pure substance
I can describe what a pure substance is, and identify a pure substance from melting and boiling point data.


- Year 10•
- AQA•
- Foundation
Pure substance
I can describe what a pure substance is, and identify a pure substance from melting and boiling point data.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- A 'pure substance' in chemistry is a single element or compound not mixed with any other substance.
- The definition of 'pure' means something different in its everyday usage.
- Pure elements and compounds have specific melting and boiling points.
- Melting point data can be used to recognise substances with specific melting points as pure substances.
Keywords
Pure substance - A pure substance in chemistry is a single element or compound that is not mixed with any other substance.
Property - A property is a feature or characteristic of a substance that can be used to classify it, or describe how it behaves.
Common misconception
Pupils can struggle to decide on the scales needed for an axis on a graph.
Suggest a suitable scale that will fit on the graph paper they will use or use a computer to draw graphs.
To help you plan your year 10 combined science lesson on: Pure substance, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Pure substance, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the States of matter unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Equipment
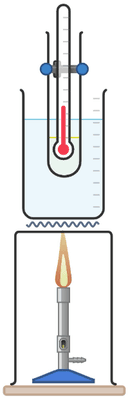
Paraffin wax, salol or other pure substances that give good cooling curve results, boiling tube, thermometer, water bath to melt substance, stop clock.
Content guidance
- Risk assessment required - equipment
Supervision
Adult supervision required
Licence
Prior knowledge starter quiz
6 Questions
Q1.A material is composed of only one type of chemical.
Q2.What is the name given to a substance found in a material in very small amounts, but which can alter the material’s properties?
Q3.Which diagram correctly represents a mixture of two metals in the solid state?
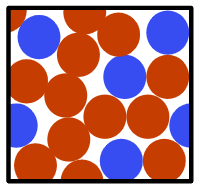
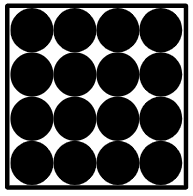
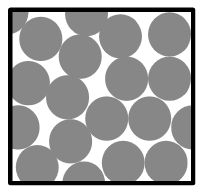
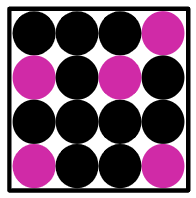
Q4.Which of these statements about mixtures are true?
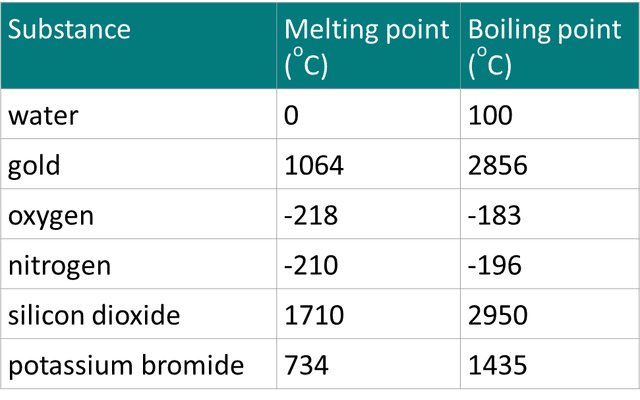
Q5.What is the highest temperature at which all of these substances are in the solid state?
Q6.Starting from a substance in the gas state, can you put the states and the temperatures at which changes of state happen in order?
Assessment exit quiz
6 Questions
Q1.A pure substance in chemistry is a single element or compound that is not mixed with any other substance. Which of the following are pure substances in chemistry?




Q2.The image shows the label of a pack of orange juice. What information on the label tells us it is not chemically pure?

Q3.Honey has a melting range of 35 to 40$$^o$$C and it decomposes before it boils. Using this information and what you know about pure substances, what makes honey an impure substance?
Q4.How is the melting point of a chemical substance represented on a graph?
Q5.A melting and freezing experiment is done with stearic acid. Put the stages of the experiment in order:
Q6.Honey can crystallise at room temperature, and starts to decompose (caramelise and smoke) at around 60$$^o$$C. How could you melt crystallised honey so this doesn't happen? Put the steps in order.


