Myths about teaching can hold you back
- Year 10
- AQA
- Foundation
State changes: evaporation and sublimation
I can explain the processes of sublimation, deposition, and evaporation, and discuss the energy changes involved in these state changes.
- Year 10
- AQA
- Foundation
State changes: evaporation and sublimation
I can explain the processes of sublimation, deposition, and evaporation, and discuss the energy changes involved in these state changes.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- Sublimation is the change from solid to gas; deposition is the change from gas to solid, both bypass the liquid state.
- Sublimation and deposition occur at specific conditions of temperature and pressure.
- Energy changes in sublimation/deposition are similar to melting/freezing but happen directly between solid and gas.
- Evaporation is when a liquid changes to gas at the surface as particles gain enough energy to overcome forces.
Keywords
Evaporation - when a particle of a substance in the liquid state at the surface, has enough energy to change to the gas state and mix with air.
Sublimation - a change of state of a substance from a solid directly to a gas; e.g. iodine.
Deposition - when a substance changes from the gas state to the solid state without being in the liquid state.
Common misconception
Pupils think that substances need to be at their boiling point temperature to evaporate.
Use plenty of real life examples of liquids evaporating in situations where pupils know that the liquid is not above its boiling point but still evaporates.
To help you plan your year 10 combined science lesson on: State changes: evaporation and sublimation, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: State changes: evaporation and sublimation, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the States of matter unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Equipment
Microscope slides, beakers, ice, warm water/electric water baths, pipettes, propanone, stop clock.
Content guidance
- Risk assessment required - equipment
Supervision
Adult supervision required
Licence
Prior knowledge starter quiz
6 Questions
Q1.Some students are revising the differences between boiling and evaporating, and they mix up their revision cards. Select all the statements below that are about evaporation.
Q2.Substances in the liquid state can evaporate at temperatures lower than their boiling point.
Q3.A cup with some hot tea is left in a cold room. The image shows you what it looks like after a day or so. What has happened?

Q4.Solid and liquid states of water are obvious in this image. What clearly indicates that there is also water present in the gas state?

Q5.Which of these are examples of reversible changes of state?
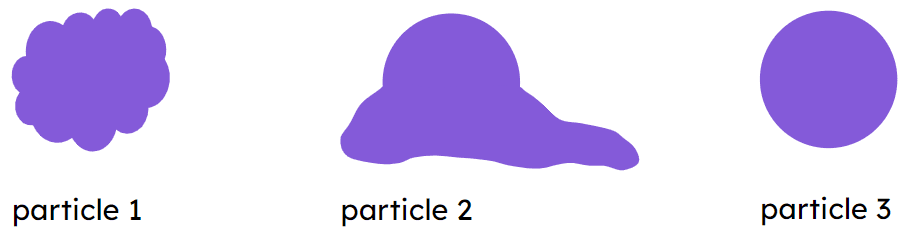
Q6.When using the particle model, which image best represents an individual particle from a substance in the gas state?
Assessment exit quiz
6 Questions
Q1.What are three crucial assumptions we have to make about the states of matter and the particle model?
Q2.Which of the three crucial assumptions means that we can explain how it is possible that particles can go directly from the solid state to the gas state?
Q3.Which of the three crucial assumptions means that we can explain how it is possible that particles can go directly from the gas state to the solid state?
Q4.For evaporation to take place, particles have to have enough energy, even if the liquid is not at boiling point. The other requirement is that such a particle is at the of the liquid.
Q5.Match the numbers in the image below with the correct state change.
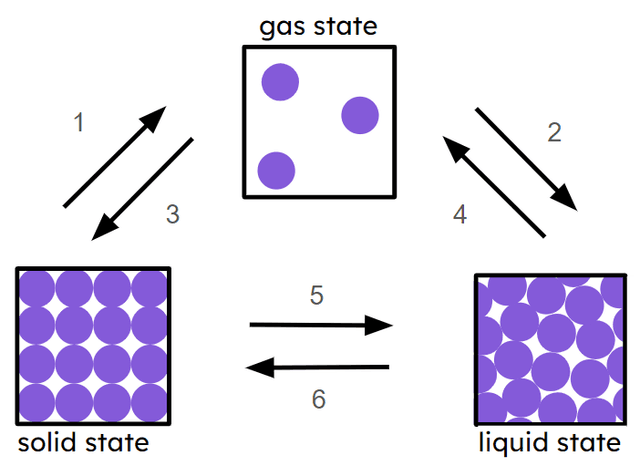
sublimation
condensation
deposition
boiling and/or evaporation
melting
freezing
Q6.Match the following key terms with their definitions.
when a substance in gas state is cooled and changes to liquid state
a change of state of a substance from a gas directly to a solid
if surface particle in liquid has enough energy to change to gas state
a change of state of a substance from a solid directly to a gas


