Myths about teaching can hold you back
- Year 10
- Edexcel
- Foundation
Modern periodic table and electron configuration
I can describe the way the modern periodic table is organised and draw the electronic configuration of the first twenty elements.
- Year 10
- Edexcel
- Foundation
Modern periodic table and electron configuration
I can describe the way the modern periodic table is organised and draw the electronic configuration of the first twenty elements.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- The periodic table is ordered by increasing atomic number (proton number).
- The periodic table is split into periods and groups.
- The position of an element in the periodic table is linked to its atomic number and the arrangement of its electrons.
- Elements can be classed as metals or non-metals according to their position on the periodic table.
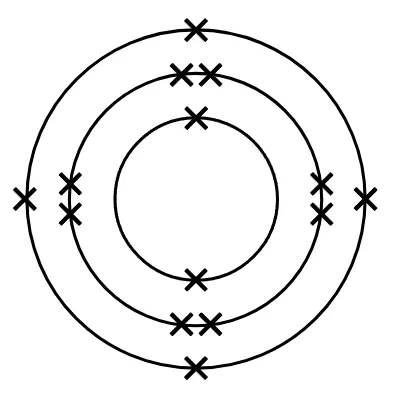
- A useful model for atoms arranges electrons in shells around the nucleus.
Keywords
Electron shell - is an energy level outside the nucleus which contains electrons.
Electronic configuration - of an atom of an element describes how the electrons are arranged in shells.
Period - is a horizontal row on the periodic table, it corresponds to the number of shells of an atom in an element that have electrons in them.
Group - is a column on the periodic table that corresponds to the number of electrons in the outer shell of an atom.
Common misconception
Pupils often confuse the atomic number and mass number with regard to the number of electrons in an atom. They also can confuse the number of electrons which can be held by each shell.
Emphasise that the lower number (atomic number/proton number) represents the number of electrons (reference to the mass being larger can help). Practise as many electron arrangements as possible.
To help you plan your year 10 combined science lesson on: Modern periodic table and electron configuration, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Modern periodic table and electron configuration, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the Atomic structure and the periodic table unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Licence
Prior knowledge starter quiz
6 Questions
Q1.Which of these subatomic particles are located in shells around the nucleus of an atom?
Q2.How many protons does an atom of carbon have?
Q3.An atom has 12 protons and 12 neutrons. What is its mass number?
Q4.True or false? You can find out how many electrons are in a neutral atom of each element by looking at the periodic table.
Q5.An element has 16 electrons. Based on its electronic arrangement, determine whether it is more likely to be a metal or a non-metal.
Q6.Place the following models of the atom in chronological order based on their development, starting from the earliest to the latest:
Assessment exit quiz
6 Questions
Q1.Which group in the periodic table contains elements with a full outer electron shell?
Q2.How many electrons can the first shell of an atom hold (maximum)?
Q3.Which of the following statements about the periodic table is FALSE?
Q4.Identify the group number for elements with electronic configuration ending in 2,7.
Q5.Write the electron configuration of calcium (separate each number by a comma ",")
Q6.Write the element's symbol below for the element represented in this electron configuration diagram.