Myths about teaching can hold you back


- Year 10•
- Edexcel•
- Foundation
Predicting states of matter
I can predict the state of matter of a substance at different temperatures using the particle model.


- Year 10•
- Edexcel•
- Foundation
Predicting states of matter
I can predict the state of matter of a substance at different temperatures using the particle model.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- There are changes in arrangement, movement and energy of particles during state changes.
- There are two types of change, physical and chemical, and these can be explained in terms of the particle model.
- The physical state of a substance can be predicted at specified temperatures.
- Forces of attraction between particles have a role in determining the amount of energy needed for state changes.
Keywords
Physical change - A change in which no new substances are formed, such as a change in state, e.g. melting.
Chemical change - When a reaction takes place and atoms or ions in the reactants are rearranged to make new products/substances.
Common misconception
Pupils often confuse physical and chemical changes. Also, pupils do not appreciate that substances can exist in any state of matter depending on temperature.
Use the phrasing 'substances in a solid state' rather than solids to avoid pupils thinking that the substance only exits in one specific state.
To help you plan your year 10 combined science lesson on: Predicting states of matter, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Predicting states of matter, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the States of matter unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Equipment
Optional : pair of magnets that have different magnetic strengths.
Licence
Prior knowledge starter quiz
6 Questions
Q1.Which of the following statements can correctly finish this sentence?: Substances in a solid state...
Q2.Which of the following statements can correctly finish this sentence?: Substances in a liquid state…
Q3.Which of the following statements can correctly finish this sentence?: Substances in a gas state…
Q4.As particles gain energy, they are able to overcome the between them.
Q5.Which aspects of a particle change when a substance’s state of matter changes?
Q6.Match the following terms to their definitions.
boiling point -
temperature where substance changes from liquid state to gas state
condensing point -
temperature where substance changes from gas state to liquid state
freezing point -
temperature where substance changes from liquid state to solid state
melting point -
temperature where substance changes from solid state to liquid state
Assessment exit quiz
6 Questions
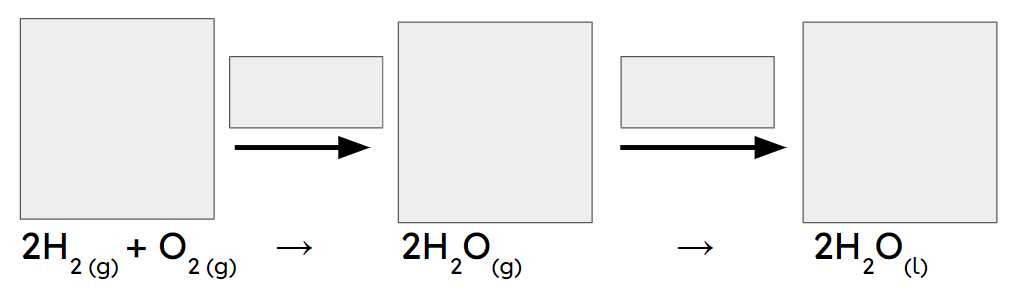
Q1.Changes of state, such as when a substance in the solid state becomes a substance in the liquid state, do not involve the formation of new substances, so these are changes.
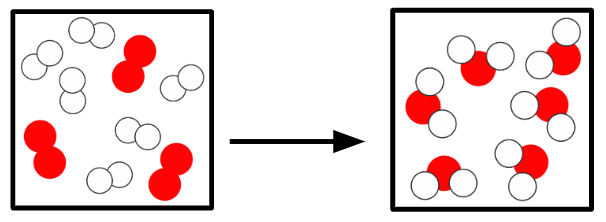
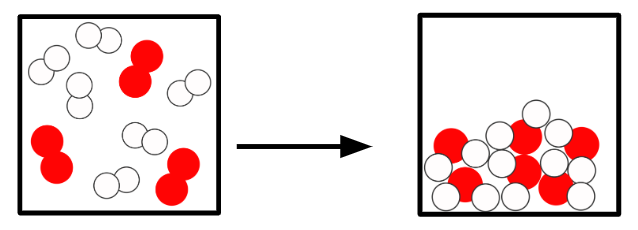
Q2.In the main image, the important information has been replaced with grey boxes. Which of the three images below is the best representation of a chemical change of substances in the same state?
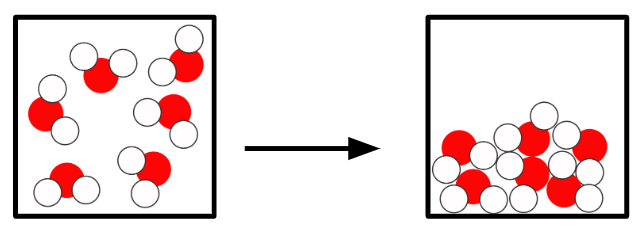
Q3.Starting from a substance in the gas state, sort the states and the temperatures at which changes of state happen in order?
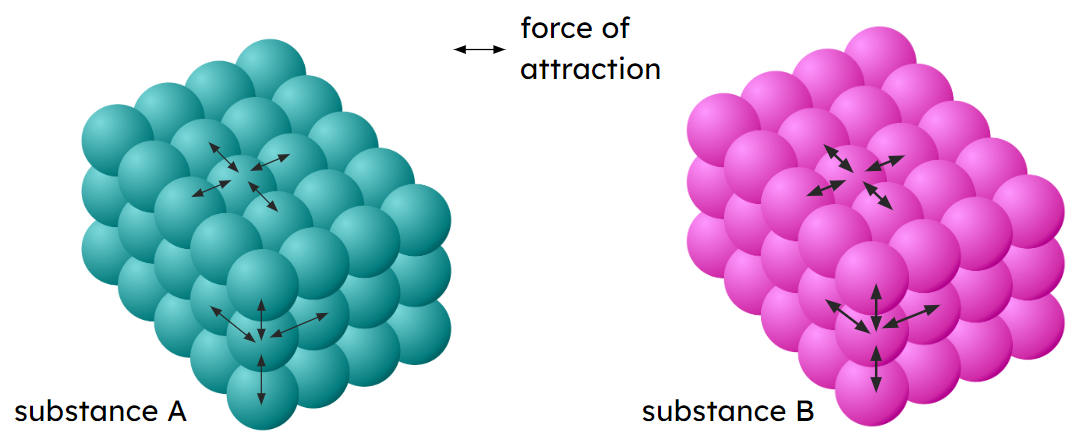
Q4.In the image below, what indicates that substance A has a lower melting point than substance B?
Q5.Three balloons are filled with neon, methane and oxygen, respectively. Why would you expect the substance in the methane filled balloon to have the highest boiling point?
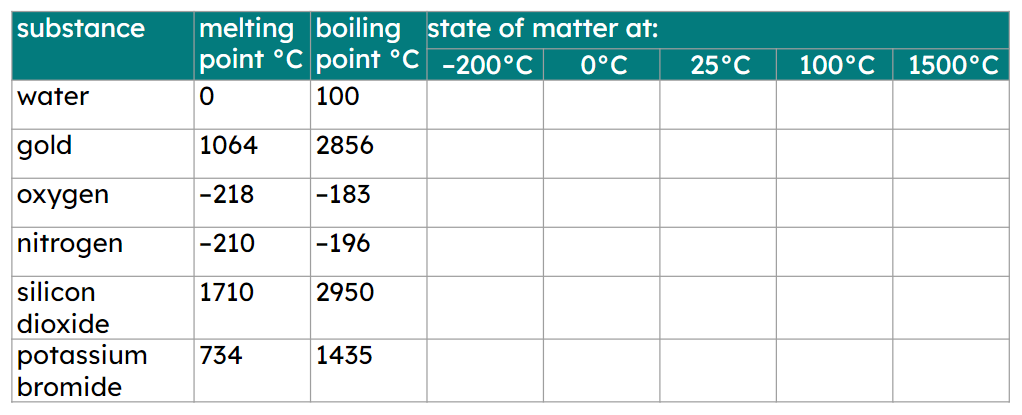
Q6.At which temperature are all these substances in a liquid state?