Bonding, structure and properties
I can explain how bonding, structure and properties are linked together and answer related long answer questions.
Bonding, structure and properties
I can explain how bonding, structure and properties are linked together and answer related long answer questions.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
- There are three different types of bonding (covalent, ionic, metallic).
- There are only two types of structure (simple and giant).
- The type of bonding and structure are what lead to properties of substances.
- Melting/boiling points are linked to intermolecular forces (simple) and bond strength (giant).
- Conductivity is linked to whether there are free moving charge carriers.
Keywords
Bonding - Refers to the force of attraction between atoms or ions that hold them together.
Force of attraction - Refers to any force that causes two or more substances to come together.
Structure - Structures in chemistry are determined by the type of bonding and arrangement of atoms or ions.
Property - A feature or characteristic of a substance that can be used to classify it, or describe how it behaves.
Charge carrier - Particles that enable electrical conductivity, such as delocalised electrons or free–moving ions.
Common misconception
Ionic substances are binary compounds. Bonding and structure are the same thing. Only bonding influences properties.
As well as multiple ions in ionic substances, there can be polyatomic ions too. Bonding informs structure, and bonding and structure together influences properties.
To help you plan your year 10 combined science lesson on: Bonding, structure and properties, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 10 combined science lesson on: Bonding, structure and properties, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 combined science lessons from the Chemistry of carbon unit, dive into the full secondary combined science curriculum, or learn more about lesson planning.

Licence
Starter quiz
6 Questions
Electrons are shared between atoms.
Electrons are transferred, so charged ions, not atoms, are bonded.
Electrons are delocalised among a lattice of metal ions.
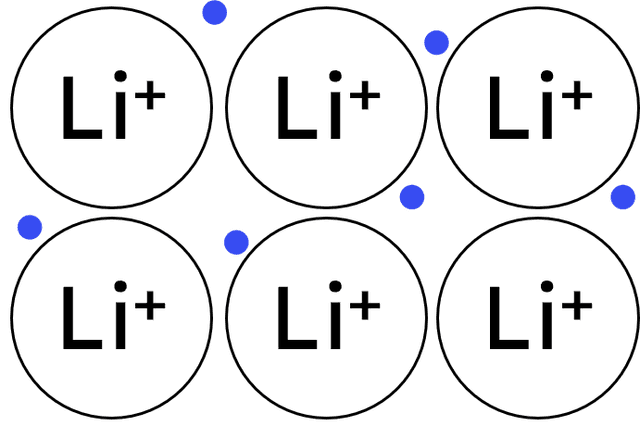
Exit quiz
6 Questions
A force of attraction between atoms or ions that holds them together.
A characteristic of a substance describing how it appears and behaves.
Particles that enable electrical conductivity.
The arrangement of particles in a substance.
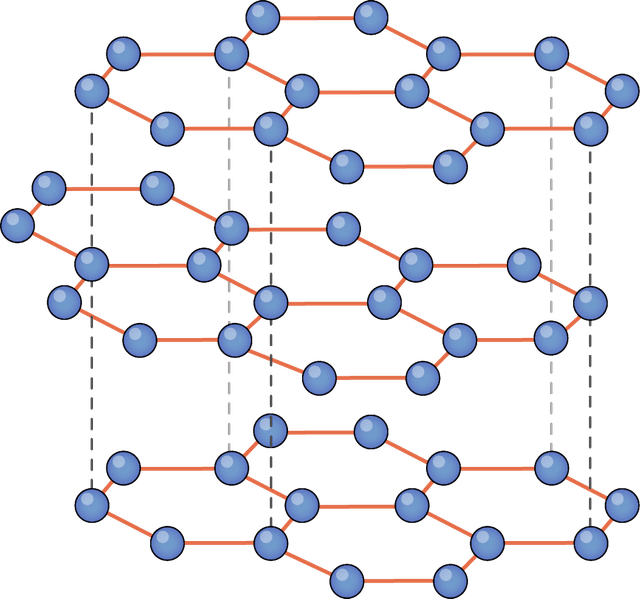
simple or giant molecular structures
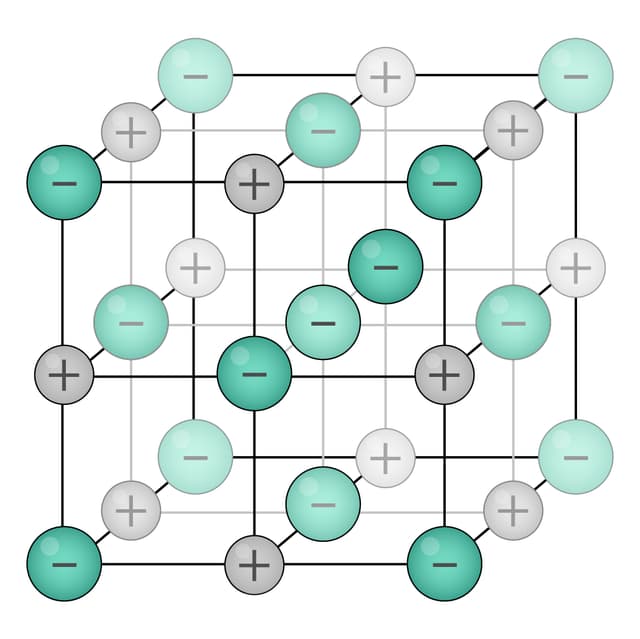
giant lattice structure
layers of positive ions in a 'sea' of delocalised electrons


