Myths about teaching can hold you back


- Year 11•
- Edexcel•
- Foundation
Unstable nuclei
I can explain why some nuclei are stable and why some are unstable.


- Year 11•
- Edexcel•
- Foundation
Unstable nuclei
I can explain why some nuclei are stable and why some are unstable.
These resources will be removed by end of Summer Term 2025.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
These resources were created for remote use during the pandemic and are not designed for classroom teaching.
Lesson details
Key learning points
- Protons have a positive electric charge of +1 and a mass number of 1.
- Neutrons have no electric charge and have a mass number of 1.
- The atomic number of an element equals the number of protons in the nucleus of each atom of that element.
- The atomic number of an element equals the number of electrons in each atom of that element.
- Atoms of an element that have different numbers of neutrons in their nuclei are isotopes of the element.
Keywords
Nucleus - the central part of an atom which contains protons and neutrons
Nucleon - the term for particles in a nucleus (protons and neutrons)
Atomic (proton) number - the number of protons within a particular nucleus
Mass (nucleon) number - the total number of nucleons within a particular nucleus (protons + neutrons)
Isotopes - atoms of the same element with differing numbers of neutrons
Common misconception
Many pupils are uncertain about the forces attracting or repelling particles inside an atom.
Extend explanations to include electrostatic forces between protons in the nucleus and introduce the strong nuclear force to explain why nuclei do not self destruct.
To help you plan your year 11 physics lesson on: Unstable nuclei, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 11 physics lesson on: Unstable nuclei, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 4 physics lessons from the Nuclear physics unit, dive into the full secondary physics curriculum, or learn more about lesson planning.

Licence
Prior knowledge starter quiz
6 Questions
Q1.Which of these are examples of natural sources of background radiation?
Q2.Which of these devices is used to detect nuclear radiation?
Q3.The figure shows a model of the atom developed by JJ Thompson. It is the model of the atom.
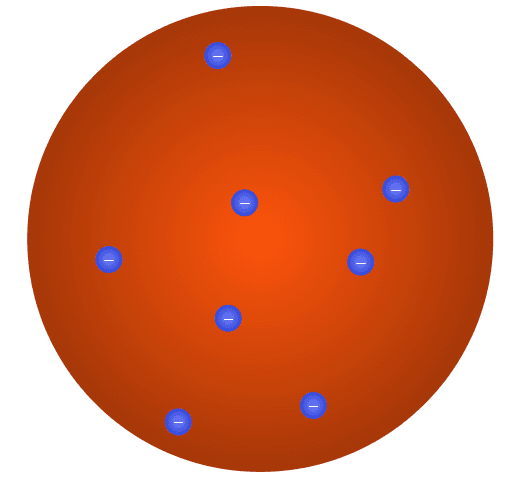
Q4.Which particles did Rutherford use in his experiment which led to the development of the nuclear model of the atom?
Q5.Which of these are conclusions from Rutherford’s scattering experiment?
Q6.Which of these refinements of the nuclear model is Niels Bohr responsible for?
Assessment exit quiz
6 Questions
Q1.Match each key word or phrase to the correct definition.
nucleus -
the central part of an atom that contains protons and neutrons
nucleon -
a proton or neutron in a nucleus
atomic (proton) number -
the number of protons within a particular nucleus
mass (nucleon) number -
the total number of protons + neutrons within a particular nucleus
isotopes -
atoms of the same element with differing numbers of neutrons

