Heating and cooling
I can explain why some changes of state are exothermic and some are endothermic.
Heating and cooling
I can explain why some changes of state are exothermic and some are endothermic.
These resources will be removed by end of Summer Term 2025.
Lesson details
Key learning points
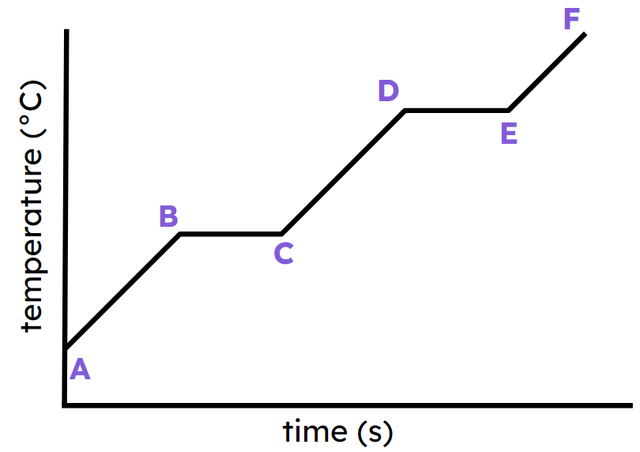
- Heating transfers energy into a substance and can cause it to melt, evaporate or boil.
- Melting, evaporation and boiling are endothermic.
- As it cools, a substance transfers energy into the surroundings by heating and it may condense or freeze.
- Condensing and freezing are exothermic.
Keywords
Endothermic - When a substance transfers energy in from the surroundings, e.g. melting, boiling and evaporating.
Exothermic - When a substance transfers energy out to the surroundings by heating, e.g. condensing and freezing.
Common misconception
Energy can appear and disappear.
Remind students about the conservation of energy.
To help you plan your year 8 science lesson on: Heating and cooling, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 8 science lesson on: Heating and cooling, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 3 science lessons from the Fuels and energetics unit, dive into the full secondary science curriculum, or learn more about lesson planning.

Content guidance
- Risk assessment required - equipment
Supervision
Adult supervision required
Licence
Starter quiz
6 Questions
Exit quiz
6 Questions

particles are in fixed positions, and vibrate more
particles becoming randomly arranged and can move
particles in liquid state move past each other and faster
energy used to overcome liquid particle attractive forces
particles in gas state use energy to move faster


