Myths about teaching can hold you back


- Year 9
The reactivity series for metals
I can use experimental evidence to organise several metals in order of their reactivity.


- Year 9
The reactivity series for metals
I can use experimental evidence to organise several metals in order of their reactivity.
These resources will be removed by end of Summer Term 2025.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
These resources were created for remote use during the pandemic and are not designed for classroom teaching.
Lesson details
Key learning points
- Some metals react with acid to produce a metal salt and hydrogen gas.
- Some metals react with water to produce a metal hydroxide and hydrogen gas.
- Some metals react with oxygen to form a metal oxide.
- The reactivity series for metals shows how vigorously metals react, compared to each other.
- The higher the position of a metal in the reactivity series, the more vigorously it is likely to react.
Keywords
Reactivity series for metals - Shows metals placed in order of reactivity with the most reactive metal at the top.
Effervescence - When bubbles of gas are produced in a liquid; another way of saying a liquid is fizzing.
Exothermic - A type of reaction in which energy is transferred from the reactants to the surroundings e.g. combustion.
Common misconception
The reactant disappears during the reaction. Acids and metals react because the acid eats away the metal.
Use word and symbol equations to reinforce the idea that during the reaction new chemicals are produced from the reactants. Demonstrate that metals less reactive than hydrogen will not react with acid.
To help you plan your year 9 science lesson on: The reactivity series for metals, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 9 science lesson on: The reactivity series for metals, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 3 science lessons from the Materials unit, dive into the full secondary science curriculum, or learn more about lesson planning.

Licence
Prior knowledge starter quiz
6 Questions
Q1.If we have a hypothesis, or if we have some experimental evidence showing a pattern, we may be able to what will happen in our next investigation.
Q2.Which of the following properties are most likely associated with metals?
Q3.Match each term to the correct statement.
acid -
a solution that has a pH of less than 7
chemical reaction -
when atoms are rearranged, and changes can be observed, e.g. bubbles
corrosion -
when a metal reduces in size due to a chemical reaction
metal -
a strong, shiny, malleable & ductile thermal and electrical conductor
non-metal -
a not strong, dull, brittle and poor thermal and electrical conductor
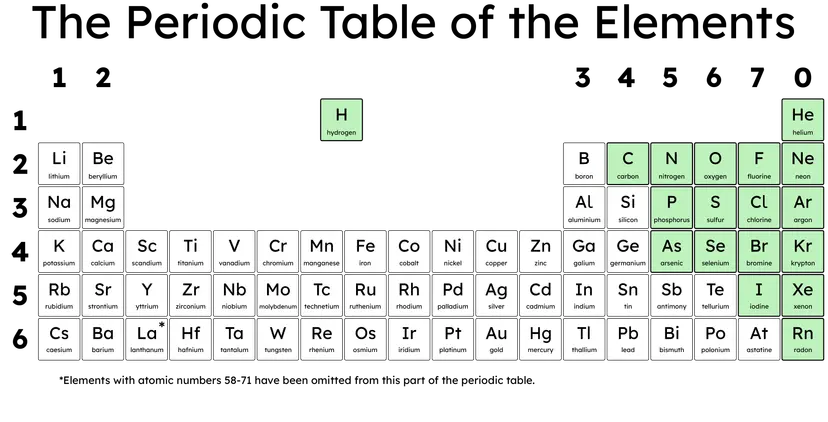
Q4.Decide whether the following statement is true or false and why this is correct: 'The elements highlighted in green in this image are all metals.'
Q5.A pupil wrote the following sentence: 'When a metal reacts, it forms salt and a gas.'
Select the statement which explains this more accurately.
Q6.Complete the word equation for the following reaction:
magnesium + hydrochloric acid -> + hydrogen
Assessment exit quiz
6 Questions
Q1.The reactivity of metals can be deduced from their reactions with certain substances. Starting with the least reactive, put the following in order of reactivity.
Q2.What happens when tin is added to water and why?
Q3.The reaction of magnesium with hydrochloric acid is irreversible. Given enough acid and time, the reaction goes to completion. MgCl$$_2$$ is very soluble. What would you see when the reaction stops?
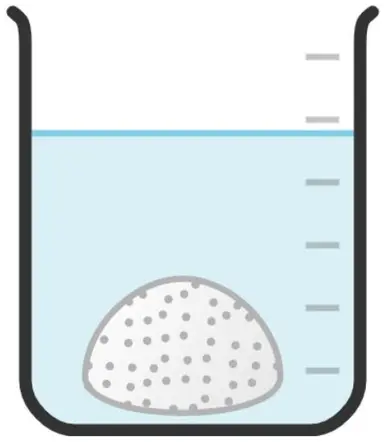
Q4.The reactivity of metals can be deduced from their method of extraction. Starting with the least reactive/easiest to extract, put the following methods of extraction in the correct order.
Q5.Match each term to the correct definition.
effervescence -
when bubbles of gas are produced in a liquid, so the liquid is fizzing
exothermic -
when energy is transferred from reactants to surroundings
irreversible reaction -
reactants form products, and the reaction goes to completion
reactivity series -
metals placed in order of reactivity; most reactive metal at the top

