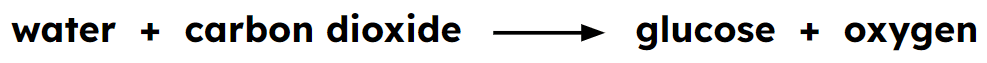Myths about teaching can hold you back
- Year 8
Conservation of mass and balanced symbol equations
I can explain the conservation of mass and write balanced chemical equations to show how the atoms of reactants in a chemical reaction are rearranged to form products.
- Year 8
Conservation of mass and balanced symbol equations
I can explain the conservation of mass and write balanced chemical equations to show how the atoms of reactants in a chemical reaction are rearranged to form products.
These resources were made for remote use during the pandemic, not classroom teaching.
Switch to our new teaching resources now - designed by teachers and leading subject experts, and tested in classrooms.
Lesson details
Key learning points
- The mass of the products in a chemical reaction is the same as the mass of the reactants.
- Word equations are a simple way of describing chemical reactions.
- Chemical formulae represent the numbers of each type of atoms in a reactant or product.
- Chemical equations represent how the atoms in the reactants rearrange to form the products.
- The arrow in a chemical equation represents the words ‘changes into’.
Keywords
Chemical formula - represents a substance using element symbols and the number / ratio of atoms of each element in the substance.
Word equation - is a way of describing a reaction using the chemical names of the reactants and products.
Balanced symbol equation - describes a reaction using a symbol equation with coefficients, which ensure there are equal numbers of atoms of each element on both sides of the symbol equation.
Coefficient - is the number placed in front of a chemical formula to balance an equation; it multiplies all the atoms in the formula and shows the ratio of substances in a reaction.
Common misconception
Pupils get confused with what numbers can be changed, many of them try to change the subscript numbers in formulae to balance equations.
Use the layout modelled in the slides to go through the examples using the 'circled method' and tell pupils that when adding more of a substance they must add all elements in the substance, e.g. 2MgO = (MgO)(MgO).
To help you plan your year 8 science lesson on: Conservation of mass and balanced symbol equations, download all teaching resources for free and adapt to suit your pupils' needs...
To help you plan your year 8 science lesson on: Conservation of mass and balanced symbol equations, download all teaching resources for free and adapt to suit your pupils' needs.
The starter quiz will activate and check your pupils' prior knowledge, with versions available both with and without answers in PDF format.
We use learning cycles to break down learning into key concepts or ideas linked to the learning outcome. Each learning cycle features explanations with checks for understanding and practice tasks with feedback. All of this is found in our slide decks, ready for you to download and edit. The practice tasks are also available as printable worksheets and some lessons have additional materials with extra material you might need for teaching the lesson.
The assessment exit quiz will test your pupils' understanding of the key learning points.
Our video is a tool for planning, showing how other teachers might teach the lesson, offering helpful tips, modelled explanations and inspiration for your own delivery in the classroom. Plus, you can set it as homework or revision for pupils and keep their learning on track by sharing an online pupil version of this lesson.
Explore more key stage 3 science lessons from the Understanding chemical reactions unit, dive into the full secondary science curriculum, or learn more about lesson planning.

Equipment
Calculators may be needed for Task A. Demo: burning magnesium - Bunsen burners, heat proof mat, tongs. Demo: thermal decomposition of copper carbonate - clamp and stand, boiling tube, Bunsen burner.
Licence
Prior knowledge starter quiz
6 Questions
Q1.What is a molecule?
Q2.In a chemical reaction, the substances formed are called .
Q3.Which of the following is a compound?
Q4.What happens to atoms during a chemical reaction?
Q5.What is the chemical formula for carbon dioxide?
Q6.A chemical symbol is a representation of a chemical reaction using the symbols of the elements involved.
Assessment exit quiz
6 Questions
Q1.What does the arrow in a chemical equation represent?